Two U.S. pharmaceutical giants, Pfizer and Moderna, are in the final phase of coronavirus vaccine development, and Oxford University is expected to start large-scale human trials of its vaccine in the U.S. this month. CBS News got an inside look at what happens during this phase, before the vaccine is approved for public use.
For Dr. Victoria Smith, who practices family medicine near New Orleans, COVID-19 is personal. She’s lost three patients to the virus.
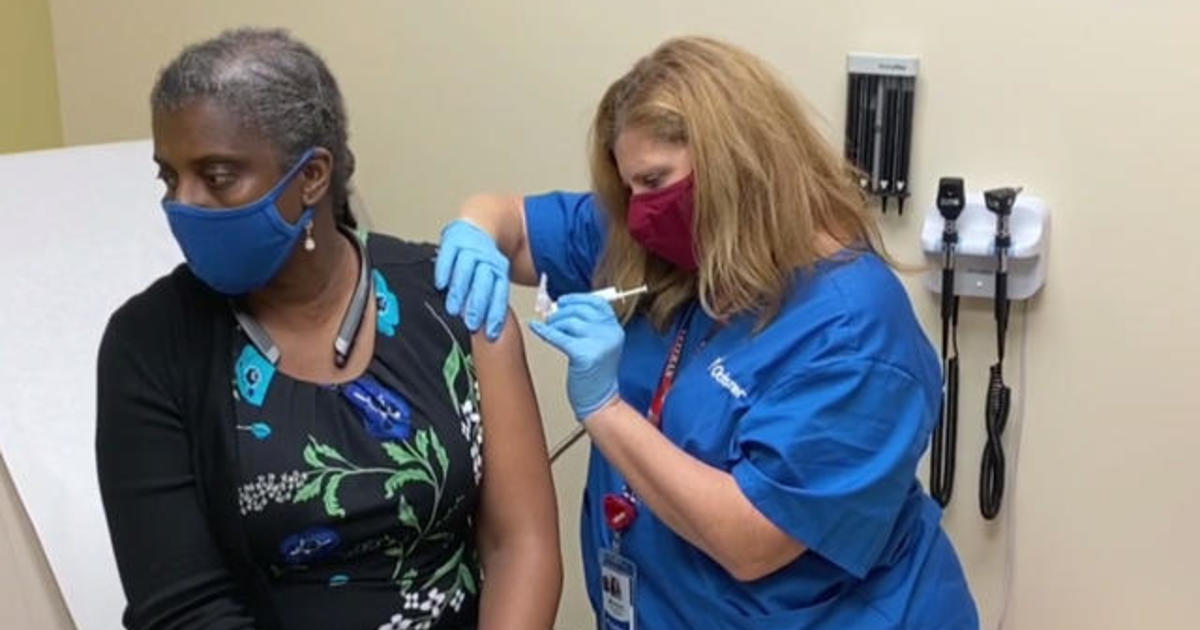
“I wanted to be part of the solution,” she told CBS News chief medical correspondent Dr. Jon LaPook. So in late July, Smith became one of the first of 30,000 participants in Pfizer’s Phase 3-vaccine trial.
“I’m not an immunologist, but by being part of a trial, I can be part of that research front as well,” she said.
She received her first dose at Ochsner Medical Center in Louisiana. It’s a double-blinded study, meaning neither she nor the researchers know whether she received the vaccine or just a placebo.
Every week, Pfizer sends packages of the vaccine and placebo to the Ochsner Phase 3 trial site. The vaccine is then stored in a freezer at a temperature of at least -60 degrees celsius (-76 Fahrenheit) to keep it stable.
Smith said she didn’t have any doubts about the trial. “I trust the process,” she said.
To be approved by the Food and Drug Administration, a COVID-19 vaccine must prevent or decrease the severity of the disease in at least 50% of vaccinated people.
“There’s no evidence that the vaccine makes the disease worse at all. So that’s really, really very encouraging,” said Dr. Kathryn Edwards, who is on Pfizer’s vaccine safety committee.
Once a week, the five members of the committee video chat to carefully look over data and scan for any adverse effects — especially serious ones that lead to hospitalizations.
“We’re very, very cautious in how we look at the data,” Edwards said. “Certainly if there were severe reactions, we would get that information right away.”
Edwards said there’s no evidence so far that the vaccine causes serious reactions, but that mild reactions are common, and should not deter anyone from getting vaccinated.
“You may have a sore arm or you may have a little tenderness or you may have a little headache or you may have a little fever. So those are things to be expected,” she said. “It’s just your immune system getting tweaked and you’re making a better response.”
Trial participants are required to self-monitor and log their symptoms on an app, recording their temperature and any reactions every day for a week after each injection. They will be asked to continue logging any symptoms on a weekly basis for two years.
On Wednesday, after 21 days, Smith received her final dose. Early trial results show a second shot boosts neutralizing antibodies that block the virus’ ability to attack our bodies.
“If you look at the immune responses in the people that got one dose and two, the two doses, their immune response was higher. So I think that… they’re probably going to need two doses,” Edwards said.
But a vaccine is not expected to mean an immediate return to the way things were.
“If the vaccine’s 50% effective, it’s not going to reduce all the disease,” Edwards said. “There’s social distancing, the masks, these things may need to continue and people will continue to need to have some thought about what their activities have and the implications that they have for the rest of the community.”