The town of Bethlehem, Pennsylvania was famous for producing steel. But the smokestacks are relics. Now, a very different kind of industry is thriving in the Lehigh Valley.
“This company was actually founded in the shadows of the old Bethlehem Steel,” said Stephen Tang, the CEO of OraSure Technologies, a biotech company that produces diagnostic tests you can do at home. As millions of Americans lost their jobs last spring, OraSure started hiring.
“You’ve had to keep all of this open all through the pandemic?” asked NPR correspondent Allison Aubrey.
“That’s right; we’ve added more people, too,” Tang replied.
OraSure pioneered the first over-the-counter HIV test kit which gives a positive or negative to a user in just minutes. It’s sold everywhere, from Walmart to CVS.
“Our experience in looking at pandemics, particularly HIV, around the world where the only way to test people was to develop a self-test, seemed to be ultimately the right answer for this pandemic,” said Tang. “We can’t have people circulating in public waiting to get tested. The best way to test people is to have the ability for anybody, anywhere, any time to test themselves.”
The at-home HIV test was a big success for a small, scrappy company based far away from traditional centers of innovation.
Tang said, “I know the community well here in Lehigh Valley. I raised my family here. This is an interesting part of the country where I think you get the best of being a Mid-Atlantic Northeast population with a good dose of Midwest values.”
And it’s a good fit with his own values. “Having immigrant parents as scientists who taught me to love science and find ways to help people,” he said.
And now, Tang and his company are trying to do for COVID what they did for HIV.
Pennsylvania-based biotech company OraSure Technologies, which produces over-the-counter diagnostic kits that you can take in the privacy of your home, is working to produce an at-home COVID-19 test that gives results within minutes.
CBS News
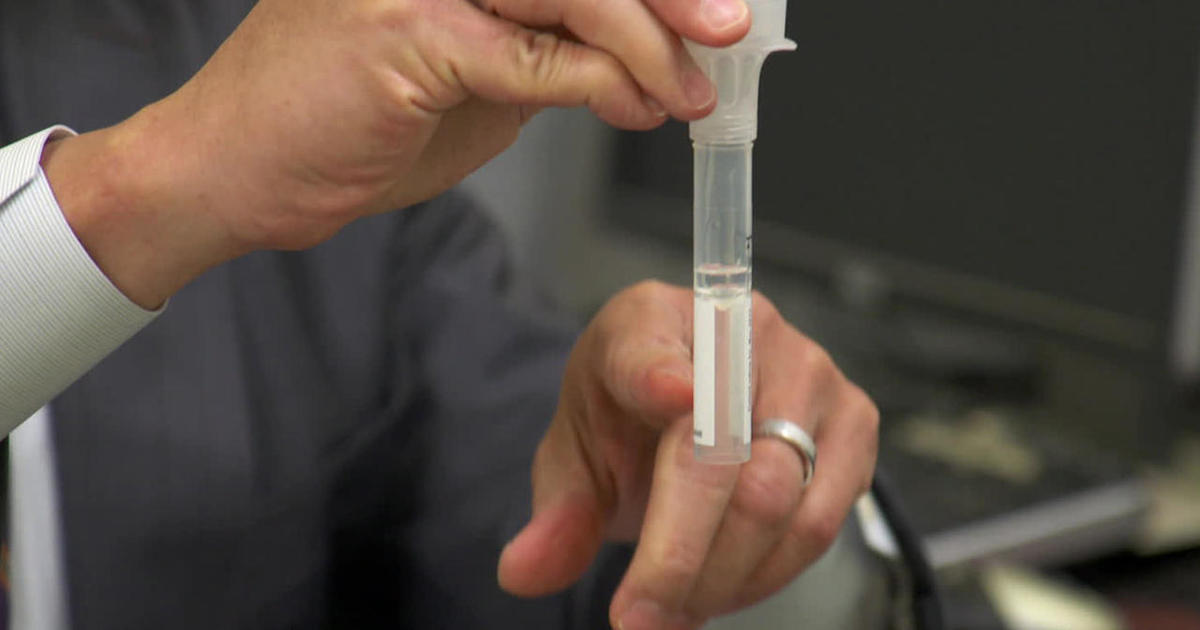
Amid long waiting times for COVID-19 testing and ongoing supply shortages, the FDA granted emergency use authorization for OraSure’s self-collection devices in late October. “We have products in the marketplace right now that allow you to sample yourself. This is a saliva collection tube and you spit into this, you send it in the mail safely.”
These kits still need to be mailed off to a lab, so they hadn’t yet solve the biggest challenge: the need for instant results.
“This is a very fast-moving virus; when somebody gets infected with the coronavirus, often by the time they are symptomatic, they have probably already begun transmitting,” said Dr. Michael Mina, assistant professor of epidemiology at the Harvard School of Public Health. As airports filled up over the holiday and cases and deaths continue to surge, he said the country would be safer if we had prioritized the kinds of tests OraSure and its competitors are developing.
“If people are waiting more than just a few days to get their results, three days, pretty much starts to make these tests almost useless for that individual. One day is already pushing it,” Mina said.
The first COVID-19 test that can be done completely at home, from Lucira Health, was approved in November, but it’s not yet available nationwide, and it requires a prescription. OraSure now hopes to have its tests on the market by the first quarter of 2021.
According to Tang, checking the control line of the test strip will give the result within an hour: “No waiting in line, no waiting for labs, no waiting for the results to come back. You own the result yourself.”
Tang knows that timing is key. The company had hoped to have its test on the market by now, but as a scientist, he also knows the importance of getting it right. “It’s not an instant gratification approach you can take to developing new products,” he said. “The science takes time to happen.”
He showed Aubrey their HIV test, which is very similar to the COVID test they’ve developed. “It means that you can test yourself anywhere, any time, and under any circumstance to get your result,” he said.
“You don’t need to call your doctor and ask for them to write you a prescription?” asked Aubrey.
“Ultimately, we are hoping this leads to people empowering themselves to find out more about themselves,” Tang said.
“What if your test is not 100% sensitive? What if it’s something a little less than that? Is it still helpful?”
“The test will be helpful, and the reason is because you allow people to be tested more frequently. Real-time information, even if it’s a little bit less precise, is more important than very precise information that you get infrequently.”
The company is aiming for at least a 90 percent sensitivity rate for its over-the-counter test.
And Dr. Mina said rapid tests are key to preventing more deaths. Even with vaccines on the way, it will likely be mid-summer before a vaccine is available to all Americans: “We don’t know how easy or hard it’s going to really be to get the vaccine out. We don’t know how durable the immunity to this vaccine is going to be. We have to have contingency plans, and we have to have tools for right now.”
For his part, Stephen Tang said the team at OraSure is geared up to give us the tools we need to keep ourselves safe right now and into the future. “Oh, boy, the people here have been working in these R&D labs pretty much 24/7 for the last nine months,” he said. “So, we’re not insisting they work those long hours. They’re working those long hours because they’re on a pursuit to really change the world.”
For more info:
- OraSure TechnologiesDr Michael Mina, assistant professor of epidemiology, Harvard School of Public Health
Story produced by Amol Mhatre. Editor: Mike Levine.